A new development in a promising lithium-ion battery alternative could make for higher-capacity, longer-lasting EV power cells. The trick? Sulfur and a bit of sugar.
Researchers at Monash University in Melbourne, Australia, have figured out a simple solution for stabilizing lithium-sulfur batteries. Lithium-sulfur is considered an emerging rival to lithium-ion, the current standard in electric vehicle batteries, with the potential to store up to five times more energy and withstand over 1,000 charge cycles. The technology also doesn’t require rare or toxic materials, hypothetically making it a more sustainable, cost-effective option.

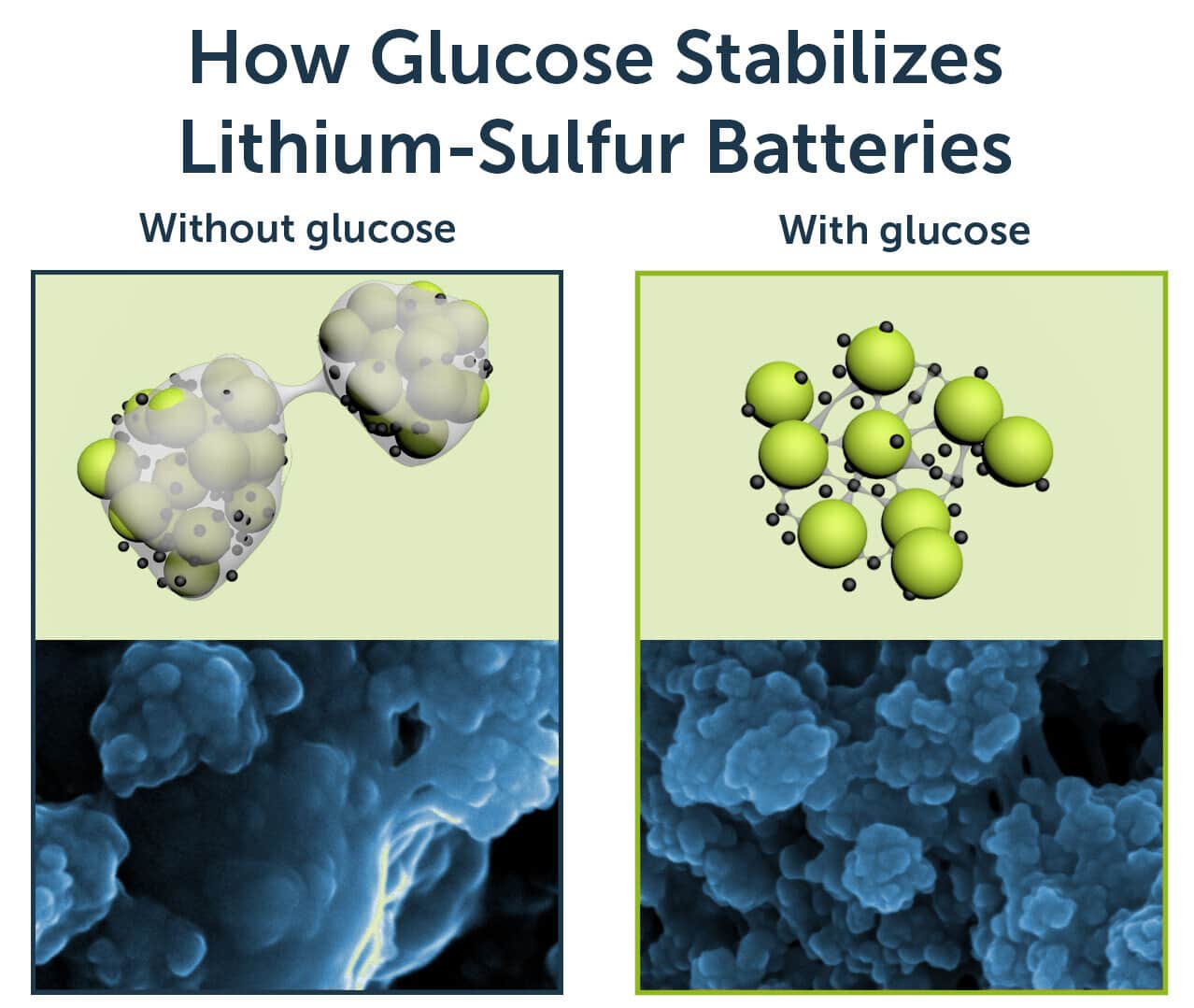
But issues with its stability have so far stood in the way of lithium-sulfur’s adoption. Batteries with this configuration are prone to deterioration due to both the way the sulfur expands and contracts and a phenomenon known as shuttling, in which the sulfur compounds contaminate the lithium anode. This not only affects the lifespan of the battery but presents a serious safety issue, too.
That’s where the new research, published in the journal Nature Communications, comes in. Using glucose like a net of sorts, the Monash team has found a way to effectively keep the sulfur in its correct place.
“In our case, the glucose we are adding in the battery is helping with the safety issue, the shuttle effect,” researcher Yingyi Huang says. That, in turn, makes it more viable for real-world use.
The most likely scenario for their use, the researcher says, would be in large, long-haul electric trucks or even agricultural drones and other forms of aviation. Lithium-sulfur is lightweight, making it an appealing option in an environment where heaviness matters more than the amount of space the battery takes up. That could mean “electric buses and trucks that can travel from Melbourne to Sydney without recharging,” lead author Professor Mainak Majumder said in a press release. Or, say, from Manhattan to Toronto.
Lithium-sulfur as a potential alternative to lithium-ion isn’t a new concept; the team’s work builds on decades of research into the subject. And the space is only growing. Analysts have pegged lithium-sulfur as a major player to watch in the global market over the next few years. The batteries would even work with today’s charging stations, making a potential switch more or less seamless.
Lithium-sulfur isn’t without drawbacks, though, even considering the latest solution. While a lithium-sulfur battery may hold onto a charge longer than its competitor, it also requires much more physical space — something that doesn’t lend itself well to many applications, like passenger vehicles or home energy storage. Performance may be superior, but that’s assuming the battery can even fit in the desired space.
Its imposing volume requirements are likely to keep lithium-sulfur from overtaking lithium-ion, which is denser and thus more compact, anytime soon, says Nikhil Koratkar, an engineering professor at Rensselaer Polytechnic Institute.
“In the context of lithium-sulfur research, what they’ve done is really good. If you’re looking at better lithium-sulfur batteries, yeah, this is one way to make lithium-sulfur batteries work better. But in general, I think lithium-sulfur will not displace lithium-ion,” he says.

“The sulfur being insulating, the only way [the battery] could work is if you’re using nano-sulfur. So everyone uses nano-sulfur, and anytime you use nanoparticles, there’s a lot of empty voidspace. The packing density of sulfur is never going to be that high.”
“If you look at the density of the sulfur cathode, it’s actually quite poor,” Koratkar says. Taking volume into account, “lithium-ion is still better.”
Just because lithium-sulfur batteries may not make it into sleek personal cars doesn’t mean the technology is useless, though. As Huang noted, a switch to sulfur could be a practical solution for larger-scale applications, like trucking and agriculture. After all, heavy duty trucks make up a bulk of the U.S.’s transportation-related greenhouse gas emissions. Electrifying could help to put a dent in that.